Connected IVD Solutions, made simple
Jump on the
digital, cloud-based, IVD train
without the hassle of building the entire backend of the application yourself with the Extra Horizon Medical Backend-as-a-Service.

INTRODUCING EXTRA HORIZON
Your go-to medical Backend-as-a-Service platform for building connected IVD solutions
Research Phase
Turn your idea into a prototype while performing all the necessary research. You will develop a comprehensive, working prototype with the help of the Extra Horizon development cluster.
Pre-market Phase
Build your first true digital medical product to pass the clinical validation stage. We provide you with all the needed infrastructure to comply with the medical security and privacy standards.
Commercial Phase
Take the next steps to bring your medical device to the market and beyond by getting your CE, FDA, ... mark. Extra Horizon is ISO13485 certified, enabling you to meet the regulatory obligations with ease.
THE BENEFITS FOR MEDICAL IVD COMPANIES
What our platform can do for your digital IVD device roadmap
AN IMPORTANT CHOICE TO MAKE
To build, or buy, your IVD solution's backend infrastructure
You have an idea for a new digital IVD product, that includes (or is a) SaMD (Software as a Medical Device). Great!
Now the big question surfaces: will you build your cloud-based backend infrastructure completely from scratch, or will you build it on an
existing, flexible and scalable backend platform like our own?
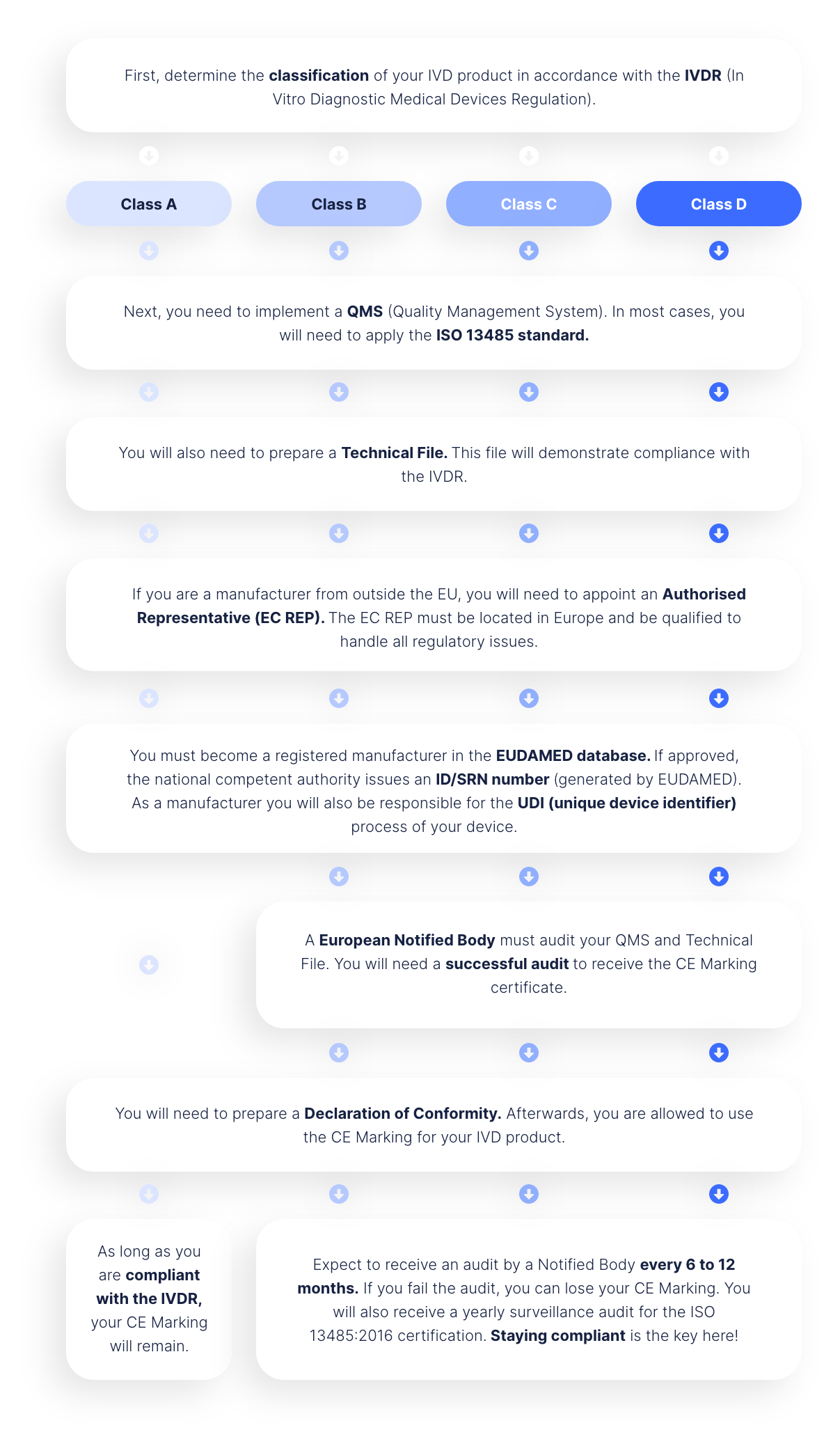
BUILDING A CE-MARKED IVD SOLUTION
The long road to obtaining the CE Mark for your IVD solution
Feeling a bit overwhelmed? We are here to help.
Not sure if Extra Horizon can support your IVD solution?
That’s the beauty of it. The Extra Horizon medical Backend-as-a-Service forms the foundation of your solution. We offer you the tools to start building the SaMD solution of your dreams in no time.
We are here to
support your idea. From connecting biosensors, to blood tests, stool analysis, home testing, genetic screening, diagnostic services, questionnaires, self assessment tests, DNA tests, COVID-19 tests and so on.
Some typical solutions we support ↓
Still in need of funding?
Most new digital health initiatives require some kind of investment. For larger, established companies these investments more often than not come from internal funds. But for startups and SMEs, most often you will require outside funding from investors.
So what are these investors looking for, and how can building on Extra Horizon help you
convince them of your IVD breakthrough?
Experience
By working with a trusted supplier, experienced in launching SaMD, you’ll have the right foundation needed to no time.
Scale
International regulatory compliance and high volume throughput solutions require specific cloud infrastructures.
Speed
Your competition is moving fast and state-of-the-art solutions are already having regulatory approvals, your time to market is key.
Secure data
Handling sensitive data, data sharing and obtaining insights requires GDPR and HIPAA requirements.