FibriCheck, built with Extra Horizon at its core, has received a 100/100 score for the DTAC
FibriCheck, a pioneering digital health app that enables users to check their heart rhythm with their smartphone, recently received the top score of 100/100 in the DTAC.
FibriCheck is just one example of the digital health solutions that uses Extra Horizon’s medical Backend-as-a-Service (BaaS) in the core of their product.
What is DTAC all about?
DTAC stands for Digital Technology Assessment Criteria for health and social care. DTAC was developed by NHSX; a government unit in the United Kingdom that drives the digital transformation of health and social care. The DTAC is a new advisory assessment criteria for commissioning digital health technologies. Essentially, it helps developers understand what it takes to make their digital health solutions suitable as an offering to the NHS.
DTAC was developed in response to developers and those making buying and commissioning decisions, looking to NHSX for clear direction on how to build and buy high-quality digital health technologies. By listening to those who were seeking to understand what the NHS is looking for when it comes to buying technologies, NHSX developed DTAC to help companies incorporate this criteria into their product development ‘by design’.
DTAC is not currently mandatory, but it brings together legislation and recognised good practice into one place - something that is very important in the medical startup field.
What is the purpose of DTAC?
DTAC aims to ensure that digital health applications meet the high NHS standards that patients deserve, with regards to clinical safety, data protection, technical security, and usability and accessibility standards.
For developers, DTAC sets the standard for what is expected to enter into the NHS and social care field.
How FibriCheck got scored for the DTAC
FibriCheck underwent a self-assessment, which was then subjected to an external review by Orcha; an external company that provides digital health assessments and certifications.

The assessment criteria focuses on 5 core areas.
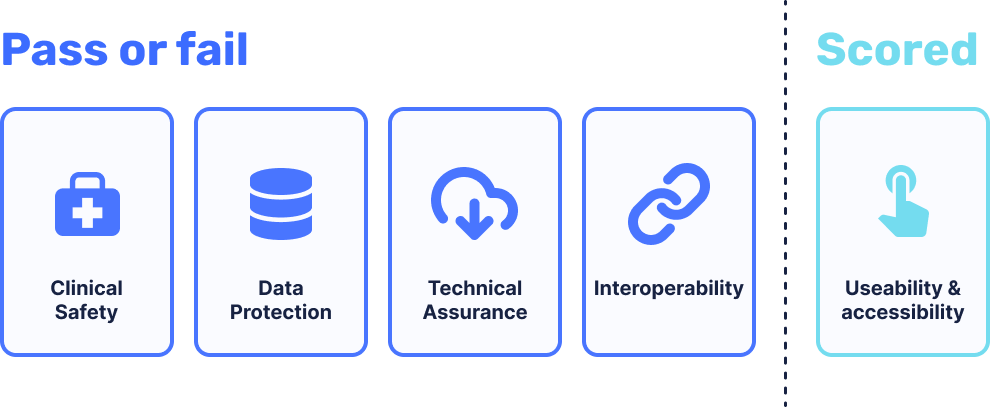
1. Clinical Safety
Products are assessed to ensure that baseline clinical safety measures are in place, and that organisations undertake clinical risk management activities to manage the risks.
2. Data Protection
Products are assessed to ensure that data protection and privacy is ‘by design’ and the rights of individuals are protected. Essentially, making sure that the protection of patient data is not an afterthought.
3. Technical Assurance
Products are assessed to ensure that products are secure and stable.
4. Interoperability
Products are assessed to ensure that data is communicated accurately and quickly, whilst staying safe and secure at the same time.
5. Usability and accessibility
Products are allocated a conformity rating, having been benchmarked against good practice and the NHS service standard.
Areas 1 through 4 are assessed on a pass/fail basis. For area 5, a combined percentage score is given.
Read more on DTAC
here.
How did Extra Horizon’s Medical BaaS help achieve this 100/100 score?
Regulatory certifications
FibriCheck was built with Extra Horizon’s medical BaaS at its core. Thus, a great deal of the regulatory burden is already taken care of. This is because our medical BaaS is certified to a wide-range of regulatory standards, such as ISO 13485:2016, ISO 27001:2017, and ISO 27701:2019, as well as being compliant with GDPR and HIPAA. In the context of the DTAC, this helped FibriCheck to achieve a passing grade in the Data Protection criteria, as it ensures that Data Protection is an integral feature of their digital medical solution.
In particular, our compliance with the ISO 13485:2016 standard helps tremendously with the Clinical Safety criteria, as this standard specifically addresses the manufacturing of medical devices, including safety criteria.
Safe and secure cloud-based platform
With digital health solutions such as FibriCheck, data often needs to be communicated from one platform to another. For example, from the patient’s smartwatch to the device of a doctor or other clinician. Therefore, it is extremely important that your solution is as secure as possible, to avoid possible data breaches and to reassure patients that the device or service will store their personal data securely. By using our medical BaaS as opposed to one that was made in-house, users are ensured that FibriCheck is built on a tried and tested, secure platform, which will handle their data safely. This feature of our medical BaaS is what helped FibriCheck pass the Interoperability criteria, ensuring easy yet safe communication of patient data.

How can Extra Horizon help your organisation with the DTAC?
By choosing to work with the Extra Horizon medical BaaS, you can relieve a huge amount of pressure if you need to adhere to the DTAC somewhere down the line. It will increase your chances of scoring a staggering 100/100 on the assessment, just like FibriCheck did. Our already-compliant, safe and secure medical BaaS will help you tick every box on your way to adhering to the DTAC. To find out more, request a demonstration, or take a look at our insights.
RECENT POSTS

FREE EBOOKS
GOT QUESTIONS?


