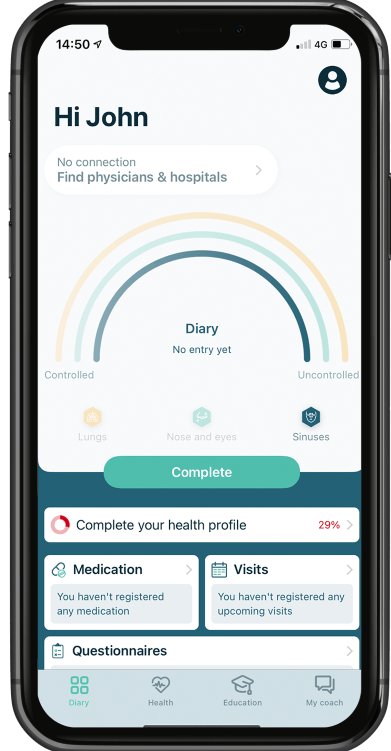
Galenus Health: a digital health solution for respiratory conditions
The mission of the Galenus Health app is to help patients to achieve a better quality of life, support doctors in their daily clinical practice, and to set up research activities. Galenus Health noticed that there is an unmet need for real-life data in this domain.
Sven Seys
Co-founder and Digital & Research Lead
Galenus Health
“Galenus Health has created a digital healthcare solution to monitor symptoms and treatment in patients with respiratory conditions. Galenus Health was founded in 2019. Today, our class I product has been launched, and the class IIa preparations are ongoing.”
Galenus Health is a fully-functional product with mobile and web-based interfaces. It functions as a symptom and care pathway tracker, a way to manage treatment adherence, and an unbiased, medically validated tool for personalised patient education. Galenus Health is a horizontal solution that can function solely for the patient (user), and can also be linked to a physician to enable them to follow-up with their patients.
Galenus is one of very few applications that has been
developed together with leading doctors in the field. It forms the basis of an international outcome registry for chronic rhinosinusitis. As such, there is a huge potential to scale the application across Europe.
Currently, the Galenus Health application is
available in 8 countries; Belgium, the Netherlands, Germany, Austria, Finland, Norway, Denmark, and the UK. They are also starting new initiatives in Sweden, France, Spain and Italy. Galenus Health is
also active in up to 25 academic centres
and several non-academic centres.
What was the problem?
In the digital health field, regulatory compliance is extremely important. Galenus Health was
faced with meeting the new MDR guidelines. Additionally, after the initial launch through EMEA, Galenus Health aspires to enter into the Canadian and US market in the next 3 years. Building a solution with global regulations in mind, such as HIPAA and FDA compliance, is crucial for its success.
Achieving regulatory compliance can be a long and difficult process. The time it takes to achieve compliance generally causes the biggest delay in the time it takes to get the product to market. This, in term, can lead to less traction when initially entering the market.
Why did they choose to work with Extra Horizon?
Extra Horizon has a proven track record when it comes to bringing medical applications to market. We have a strong regulatory foundation, with our medical Backend-as-a-Service (BaaS) being
GDPR and HIPAA compliant
from a Privacy and Security perspective, and also operating under the necessary
ISO certifications
for building Medical Software, following the IEC 62304 standards. Using a medical backend supplier that already has the necessary certifications, like Extra Horizon, does not mean that a company becomes automatically certified. However, it does mean that it is easier and quicker for a company to achieve their own certifications, as did Galenus Health.
How do they benefit from working with Extra Horizon?
Galenus Health was built with our medical BaaS at its core. This covers the necessary privacy and security requirements, and helps create the foundation for building a Class IIa regulated device.
Galenus Health had discussions with Extra Horizon’s Chief Compliance Officer, Jo van der Auwera, who helped bring the regulatory compliance requirements to a successful conclusion. As the Extra Horizon backend is built according to MDR requirements, this means that all documentation is MDR compliant. This includes all documentation concerning requirements and testing records, Data Processing Agreements, and versioning and end-of-line record keeping.
Although Galenus Health also needed to achieve their own regulatory certifications, this process was much quicker since the bulk of the underlying building blocks of their solution is already certified by a
Trusted Supplier.
What is the added value?
The main benefit of working with Extra Horizon is that the solution was ready to launch in less than 9 months. As Galenus Health was built on Extra Horizon’s compliant medical BaaS, this meant it was quicker and easier for Galenus Health to achieve their own regulatory compliance certifications.
Without this compliant backend, the process to become certified as a medical device would have taken much, much longer. This is because our backend already covers the necessary privacy requirements and provides a foundation for building a Class IIa regulated device. From day one, your system is already built with scalability in mind by a dedicated team of experts. This removes a lot of design anxiety and helps to achieve your end goals more efficiently.
The speed of this process is what ultimately allowed Galenus Health to become active in so many different countries so quickly.
Why working with Extra Horizon is the way forward for Galenus Health
A cloud-based platform means that there is no longer a need to install and maintain software solutions on the premises. This offers a flexible and secure dashboard solution for the doctors who use the application, which can be used from anywhere at any time.
Some hospitals were initially suspicious of cloud-based solutions, but the
secure, ISO compliant backend
provided by Extra Horizon reassured users that the platform is safe and secure for collecting patient data. This enabled hospitals to harness the power of the cloud for their benefit.
Want to learn more about our solutions?
Send us a message with your question(s)!
RECENT POSTS

FREE EBOOKS
GOT QUESTIONS?